With the development of science and technology, color measurement instruments are also constantly evolving. Different types of color measurement instruments vary in their working principles and measurement accuracy. Currently, color measurement devices are mainly divided into two types: colorimeters and spectrophotometers. Although both instruments are used to measure the color of samples, they differ in terms of working principles, wavelength ranges, sensitivity, cost, and applications. This article provides a brief introduction to the differences between colorimeters and spectrophotometers in color measurement instruments.
What are spectrophotometers and colorimeters?
Common contaminants in tap water include gases, enzymes (such as nucleases and proteases), microorganisms (such as bacteria and viruses), organic matter, colloids, and inorganic ions. The design of water purification systems aims to effectively remove specific types of contaminants for particular applications. Key differences between various purification systems also include tank capacity, water production rate, water quality standards, system scalability, maintenance frequency, and whether remote water extraction is supported.
What are the most common contaminants in laboratory water
What is a colorimeter
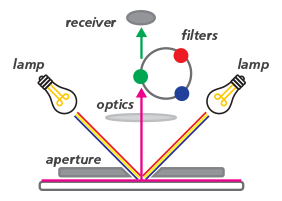
A colorimeter is an instrument that measures the color intensity of a sample by comparing it to standard colors. Its principle involves shining light of a specific wavelength onto the sample and measuring the amount of light absorbed or transmitted by the sample. The colorimeter then compares the light intensity of the sample to that of the standard light to determine the sample's color. A colorimeter uses an internal light source to illuminate the sample's surface. When the light reflects back to the device, it passes through three filters: red, green, and blue. After passing through the three-color filters (RGB, red-green-blue filters), the filters can extract the three-stimulus (RGB) values, which match the colors perceived by the human eye.
What is a spectrophotometer
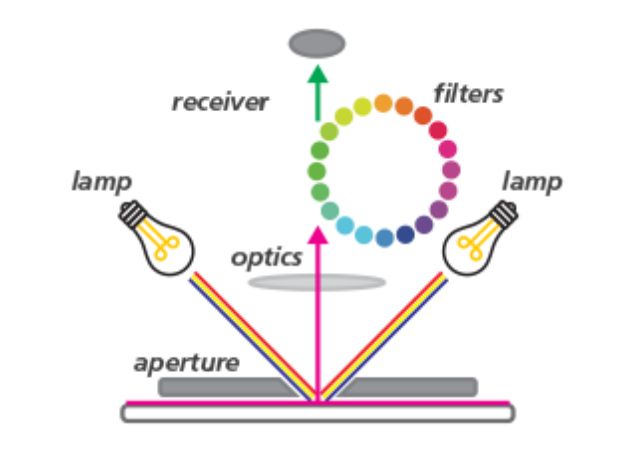
A spectrophotometer is an instrument used for physical sample analysis through full-spectrum color measurement. It offers extremely high precision and provides a wide range of data. Its principle is similar to that of a colorimeter, except that the RGB color filters are replaced with a larger number of filters to obtain full-spectrum data. By shining light of different wavelengths onto the sample, the instrument measures the amount of light absorbed or transmitted. Spectrophotometers enable more complex color measurements and can capture greater color detail.
Types of Colorimeters and Spectrophotometers
Types of Colorimeters
Colorimeters are essential for objectively and accurately measuring color. Different types of colorimeters can measure color at varying depths and intensities. Types include:
Gloss Meters:
Used to assess the surface gloss of materials such as coatings, plastics, and metals, helping to determine their quality and appearance.

Portable Gloss Meter
Measurement Time : 1.5 seconds
Inductor : Silicon photodiode
Measuring Angle 20° 60° 85°
Benchtop Colorimeter:
Color photometers measure how color is transmitted and reflected.
Benchtop Colorimeter
Integrating Sphere Size : Φ154mm
Light Source : 360 nm to 780 nm
Spectrophotometric : Mode Concave Grating
Sensor : 256 Image Element Double Array CMOS Image Sensor
Photometers: Color photometers measure how color is transmitted and reflected.
Types of Spectrophotometers
There are several types of spectrophotometers:
Atomic Absorption Spectrometers:
These analyze metal elements in materials by measuring the absorption of radiation from atomic vapors.
Fluorescence Spectrophotometers:
These spectrophotometers scan the fluorescence spectra of liquid fluorescent labels, typically used in scientific research, clinical testing, and food testing.
Infrared Spectrometer:
This machine measures the absorbance level of materials at wavelengths below 760 nm.

NIR Spectrophotometer
Wavelength Range : 900nm-2500nm
Wavelength Accuracy : ≤0.2
Wavelength Reproducibility : ≤0.05
UV Vis Spectrophotometer:
This device analyzes materials using visible and ultraviolet wavelengths, quantitatively measuring absorbance.
VIS Spectrophotometer:
This device analyzes visible wavelengths, measures absorbance, and performs quantitative analysis.
How do they work?
Colorimeter
Colorimeters work according to the Beer-Lambert law, whereby the concentration of solute is proportional to absorbance. Light emitted by the light source passes through a filter to become a specific wavelength, which then passes through the sample solution. A photodetector on the other side of the solution measures the amount of light absorbed, and the results are displayed on a digital screen via a processor.
Spectrophotometer
A spectrophotometer works by measuring the absorption of light at different wavelengths by a substance. The light source emits white light, which is then split into different wavelengths by a monochromator. The selected wavelength of light passes through the sample solution, with some of the light being absorbed by the solution. The light that passes through the solution is captured by a photodetector and transmitted to a processor for calculation of the absorbance, with the results ultimately displayed. The concentration of the substance in the solution can be calculated based on the absorbance.
Colorimeters vs. Spectrophotometers: Pros & Cons
Colorimeters
Advantages:
Colorimeters focus on tristimulus values, avoiding unnecessary full-spectrum data.
They are more portable, have a simple structure, and are easy to move or use on-site.
Colorimeters work quickly and are suitable for applications that require a fast response, such as assembly lines.
Disadvantages:
Colorimeters cannot provide comprehensive data and cannot measure spectral information or colorant intensity.
Functionality is limited, primarily used for comparison with pre-set samples, unsuitable for research or product development.
They cannot identify metamerism, such as color changes under different lighting conditions.
Spectrophotometers
Advantages:
Spectrophotometers are highly comprehensive, capable of measuring spectral data that colorimeters cannot capture.
They are versatile, allowing adjustment of light sources and observer settings to meet different needs.
When paired with powerful software, they provide more comprehensive data analysis.
Suitable for various sample types, including powders, liquids, and transparent materials, with portable versions available.
Provides results quickly, with some models taking only four seconds.
Helps achieve consistent color, ensuring brand consistency and process control.
Ideal for precise analysis, suitable for research environments with high precision requirements.
Disadvantages:
More complex to operate, not ideal for factory environments.
Spectrophotometers, with their precise and extensive information range, are typically more expensive than colorimeters.
Main difference between a colorimeter and a spectrophotometer
There are many similarities between colorimeters and spectrophotometers, but there are still significant differences between the two. The biggest difference lies in their functions and applications. Spectrophotometers are powerful tools that can provide more in-depth color measurements than colorimeters, such as spectral data. Therefore, they are primarily used for precise measurements in research and development or laboratory settings. In contrast, colorimeters are simpler to operate and are more commonly used in manufacturing and production, such as for quality control.
| Colorimeter | Spectrophotometer |
| Principle | Measures the intensity of light absorbed or transmitted by a sample at a single wavelength. | Measures the amount of light absorbed or transmitted by a sample at multiple wavelengths |
| Wavelength range | Narrow band, typically 400-700 nm | Wavelength range typically 200–800 nm or wider |
| Accuracy | Lower accuracy, typically +1-0.02 | Higher accuracy, typically ±0.001–0.005 |
| Cost | Cheap | Expensive |
| Portability | More portable | Less portable |
| Sample type | Can only measure solid or liquid samples in solution. | Can measure solid, liquid, or gas samples. |
Conclusion
In general, colorimeters are suitable for quick and easy color measurement, while spectrophotometers offer higher precision and comprehensive data analysis. The choice of equipment depends on the specific application requirements, budget, and data accuracy requirements. Understanding the differences between them can help you make more appropriate decisions in color management.

 English
English















