High performance liquid chromatography (HPLC) and gas chromatography (GC) occupy an important position in chromatographic analysis and are the most commonly used analytical methods in our drug analysis practice. They are of great significance for the determination of drug content and the examination of related substances. Today, we will continue to learn more about HPLC and hope that it will be helpful to you.
What is High Performance Liquid Chromatography?
HPLC is an analytical technique that separates, identifies or quantifies each mixture component. In simple terms, HPLC is a chromatographic method for separating and determining samples.
An HPLC system generally consists of an infusion pump, injector, column, detector, data logging and processing unit. The infusion pump, column and detector are the key components.
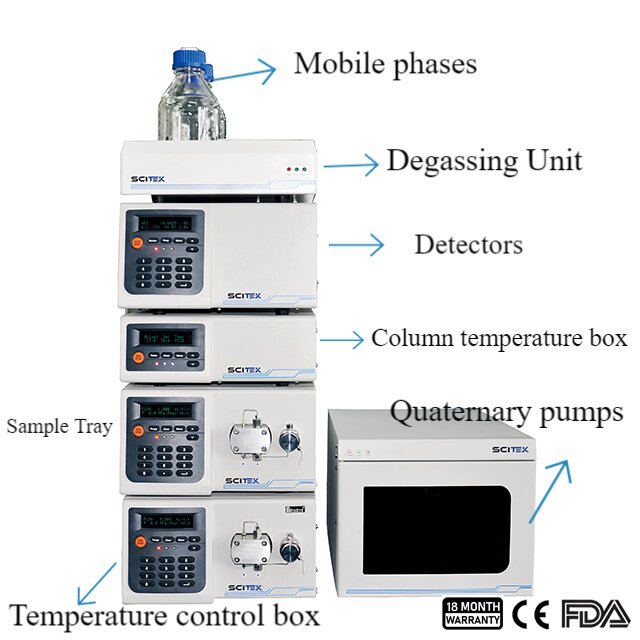
Common terms used in HPLC system
Resolution: The ratio of the difference between the retention time of two neighboring peaks to the average peak width. Also called resolution, it indicates the degree of separation of two adjacent peaks.
Tailing Factor: A parameter that evaluates peak shape by calculating the ratio of peak width at 5% peak height to the distance from the peak apex to the leading edge. The purpose is to ensure the chromatographic separation effect and measurement accuracy, commonly used as T to indicate.
Signal to Noise: The ratio of signal to noise in liquid chromatography. It is an important index to measure the performance of the liquid chromatograph and is used to evaluate the resolution and sensitivity of the instrument in separating and detecting samples.
Area/Height refers to the area size or height of the peaks in liquid chromatography, which can reflect the content and relative concentration of the components in the sample, commonly used in quantitative analysis.
Plates: Also know as column efficiency, one of the column parameters of chromatography. Employed to numerically articulate the separation effectiveness of the chromatographic column.
Retention Time: The interval from the beginning of sample injection to the chromatographic vertex of a component is called the retention time of the component. That is, from the injection to the time interval after the column when the concentration of a component appears to be great. Commonly used in qualitative identification.
t: The retention time of a component with a partition coefficient of 0 is called the dead time. Air or methane are usually considered components and are used to determine the dead time.
How does HPLC work?
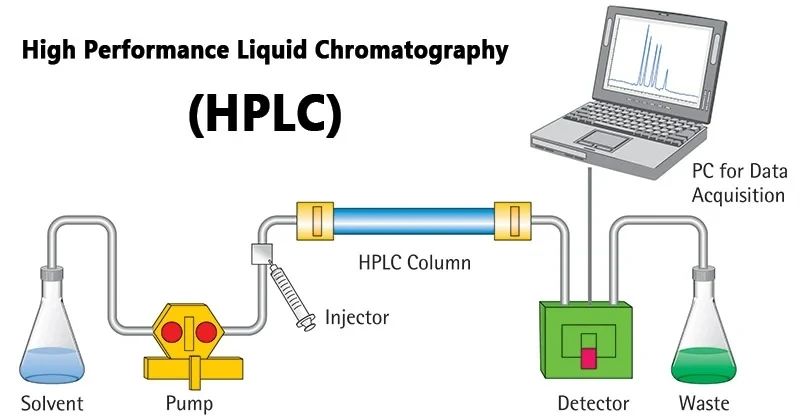
Simple summary: mobile phase → pump → injector → column → detector → waste drum
Some of you are not sure about the direction of the column connection in different HPLC system (the order of the modules in the instrument is not always fixed).
Usually, the whole flow process is fixed: Firstly, the mobile phase is extracted from the mobile phase vial by the high-pressure infusion pump and enters the injection system. In this system, the sample is added to the flow path by switching a six-way valve. At this point the sample is separated and analysed by the column and the subsequent detection system is transmitted in real time to the software for data profiling.
Infusion System
Infusion pump is one of the most important parts of the HPLC system, mainly composed of liquid storage device, filter, degassing device and high-pressure infusion pump.
Liquid storage device: The solvent should be selected chromatographic grade;
Filter: Mobile phase needs to be pre-filtered by 0.45um membrane before entering the system to avoid clogging the column, especially the buffer salt prepared with inorganic salt;
Degassing device: Mobile phase is degassed by heating, suction or helium absorption to remove air bubbles. It prevents the bubbles released when the mobile phase flows out of the high-pressure column from entering the detector and causing a sharp increase in noise, which affects the detection;
High-pressure infusion pump: Its performance directly affects the reliability of the analysis results. Try to choose the infusion pump with smooth pressure and small pulse.
* Infusion pumps are categorized based on their operational modes into pneumatic pumps and mechanical pumps. In the daily analysis process, we often use the pneumatic pump in the binary pump and quadruple pump. Binary pumps have a higher mixing precision and a more accurate ratio of mobile phase mixing. Quaternary pumps are more economical.
Sampling System
Sampler: Currently, the six-way injection valve and autosampler are more commonly used in HPLC systems. It has good sealing and small volume.
Six-way injection valve is divided into the main road and bypass, which usually need to be switched to the bypass by the quantitative ring quantitative sampling and then cut back to the main road by the mobile phase to promote the samples obtained in bypass into the system, to complete the sampling.
Corresponding to the left figure is the ready state of the bypass in the hplc system; corresponding to the right figure is the injection state.
The mobile phase and the column form a closed loop; the sample is injected from the inlet and enters the quantitative loop, and the excess is discharged into the waste liquid bucket.
After the sample enters completely, change the connection of the six-way valve. Currently, the mobile phase is connected to the quantitative ring, and the mobile phase pushes the sample into the column.
At this time, the six-way valve changes the connection mode again and returns to the initial state, and the sample to be tested is separated and detected by elution of the mobile phase.
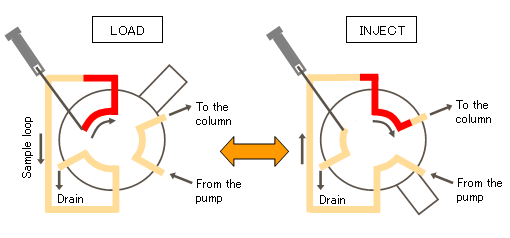
Schematic diagram of the construction principle of the six-way inlet valve
Separation System
The separation system includes includes the column, stationary phase, and mobile phase.
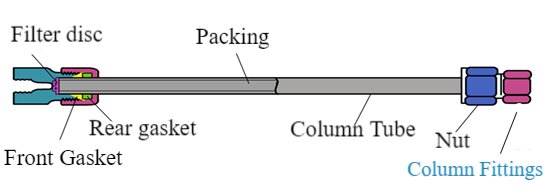
Chromatographic column structure
Chromatographic column: It is the core part of the separation system, consisting of column tube, pressure cap, ferrule (sealing ring), sieve plate (filter), joints, screws and so on. It has the following main types:
| Type | Feature |
| Reverse Phase Chromatographic Columns | Carrier bonded to a non-polar group, commonly used fillers: octadecylsilane-bonded silica gel (C18 column), octylsilane-bonded silica gel (C8 column) and phenyl-bonded silica gel, etc. |
| Normal Phase Columns | Columns filled with silica gel filler, or silica gel bonded to polar groups. |
| Ion Exchange Columns | Columns packed with ion exchange fillers, divided into cation exchange columns and anion exchange columns. |
| Chiral Separation Columns | Columns filled with chiral fillers. |
Parameters affecting the separation performance of the column:
Own parameters: Length of the inner diameter of the column, filler properties, particle size and particle size distribution, pore size, surface area, etc.
Column temperature: Column temperature should not exceed 60 ℃. The increase in column temperature will affect the peak of impurities; theoretically, the higher the column temperature, the faster the peak of each substance, which is conducive to improving the column efficiency and increasing the degree of separation.
pH: Residual silica hydroxyl unclosed silica gel column, mobile phase ph should generally be between 2 ~ 8; residual silica hydroxyl has been closed silica gel, polymer composite silica gel or polymer column, mobile phase ph is generally greater than 8 or less than 2.
Mobile Phases :Methanol-water and acetonitrile-water systems are commonly used for reversed-phase chromatography. Acetonitrile-water systems are preferred for detection at the end of the UV wavelength. Whenever possible, buffer salts should not be used in the mobile phase, and when they are used, they should be used at low concentrations.
Detection System
UV detector: One of the most widely used detectors in HPLC systems, it is responsive to most organic compounds.
Characteristics: High sensitivity; wide linear range; insensitive to changes in flow rate and temperature of the mobile phase; wavelength selectable, easy to operate;
Limitations: Solvents with certain UV absorption cannot be used as the mobile phase, and the operating wavelength of the absorption detector cannot be smaller than the cut-off wavelength of the solvent.
Specifically, it can be divided into VWD and DAD detectors
Photodiode array detectors
Differential refractive detector:
A concentration-based universal detector in the HPLC system that responds to all solutes;
Characteristics: Low sensitivity, temperature sensitive, cannot be used for gradient elution;
Fluorescence detector: The most sensitive and highly selective detector, the minimum detection concentration can reach 0.1ng/ml, suitable for trace analysis, but the linear range is not as wide as the UV detector, and can detect compounds that can produce fluorescence.
Comparison of HPLC and GC
Object of analysis
GC: Applicable to samples that can be gasified, with good thermal stability and low boiling point, accounting for 20% of organic matter
HPLC: Applicable to the samples that can be made into solution after dissolution. Samples with high molecular weight, difficulty in gasification, poor thermal stability, and high boiling point can be detected.
Mobile phase
GC: Mobile phase is inert, with no interaction between the components and the mobile phase; the mobile phase only transports the sample through the stationary phase, and the components only interact with the stationary phase.
HPLC: Mobile phase is liquid, and the mobile phase and the components of the interaction between the participation and influence of chromatographic separation for the optimization of chromatographic conditions and the improvement of separation provide a new idea.
Operating conditions
GC: Heating operation
HPLC: Room temperature (column temperature is generally not more than 60 ℃), high-pressure
Sensitivity
The sensitivity of gas-phase detection is greater than that of liquid-phase
Application range
The application range of liquid phase is much larger than liquid phase
Comparison of Normal Phase HPLC and Reversed Phase HPLC
The two most common variants of HPLC are normal-phase HPLC and reversed-phase HPLC.
Normal phase HPLC
HPLC columns are packed with minute silica particles and polar solvents like hexane.
Typical columns have an inner diameter of 4.6 mm or less and a 150 to 250 mm length.
Non-polar compounds in the mixture will pass through the column more quickly.
Reversed-phase high performance liquid chromatography
The HPLC column is the same size. The column is filled with silica particles modified to make them non-polar.
Using a polar solvent, such as a mixture of water and an alcohol, such as methanol. Polar compounds in the mixture will pass through the column faster because of the strong attraction between the polar solvent and the polar molecules.
Reversed-phase HPLC is the most commonly used form of HPLC.
Note on the use of high performance liquid chromatographic columns
Match the type of high performance liquid column head and the stainless steel capillary joints: If the joints and the depth of the column head do not match, there will be liquid leakage or the dead volume will be too large.
Matching when switching between different solvents
If the solvent stored in the column and the solvent stored in the instrument system does not match the mobile phase, they should be switched before use.
Especially when the mobile phase contains buffer salts, such as the preservation of the solvent is a pure organic phase or a high proportion of organic phase, if directly connected to the chromatographic column, it will lead to buffer salts crystal precipitation in the column, and even lead to the new column of permanent, irreversible damage.
Equilibration and aging of new columns before use.
pH range
Generally, the pH range of chromatographic columns is 2-8.
HPLC Column storage
The mobile phase used for 1-2 days short-term storage is best without buffer salts.
Pure methanol or acetonitrile is generally only recommended for long-term storage of reversed-phase columns, or 80% organic phase storage is also a good choice and sufficient to avoid bacterial growth.
CN(cyanide)-based columns are unstable in organic polar solvents and are suitable for cold storage in an aqueous phase.
The maximum operating temperature is 60°C, the optimum temperature is less than 40°C.
Separation of alkaline substances, the mobile phase pH value used should be one unit above the measured PKA
Applications of HPLC
HPLC has developed into a universally applicable method and as such it can be used in almost all areas of chemistry, biochemistry and pharmaceuticals.
Analysis in the pharmaceutical field
Examination of synthetic polymers
Identification of contaminants in environmental analysis
Quantification of drugs in biological matrices
Extraction of valuable products
Quality assurance and control for industrial products and fine chemicals
Purification and isolation of biomolecules like enzymes or nucleic acids
Treatment of water for purification purposes
Concentration of trace components before analysis
Chromatographic technique involving ligand exchange
Protein separation through ion exchange chromatography
High pH anion exchange chromatography of carbohydrates and oligosaccharides
HPLC Troubleshooting Guide
High performance liquid chromatography (HPLC), as a highly sophisticated instrument, is prone to several tricky and minor problems if not operated correctly during use. The most common problems are column pressure, drift, and peak anomalies. Therefore, how do you solve the problem quickly when facing a faulty chromatograph?
Excessive pressure
Sudden increase in pressure is the most common problem in the use of HPLC, generally for the following reasons:
| Causes | Solutions |
| Blockage in the Flow Path | Disconnect the inlet to the vacuum pump so the PEEK tube is below the solvent bottle and see if the liquid drips freely. The solvent filter head is clogged if the liquid does not drip or drips slowly. | Soak it in 30% nitric acid for half an hour and rinse it with ultrapure water. If the liquid drips freely, the solvent filter head is normal. |
| Open the Purge valve so the mobile phase does not pass through the column; if the pressure does not drop significantly, the filter white head is clogged. | Remove the filter whitehead and sonicate with 10% isopropyl alcohol for half an hour. The filter white head is normal if the pressure drops below 100 PSI. Check again. |
| Remove the outlet end of the column; if the pressure does not drop, the column is clogged. | If the buffer salt is clogged, flush with 95% water until the pressure is normal.
|
| Incorrect Flow Rate Setting | Reset to correct flow rate. |
| Incorrect Mobile Phase Ratio | Replace with a less viscous solvent or reset ratio. |
| System Pressure Zero Point Drift | Adjust zero point of pressure sensor. |
Too low a pressure
| Causes | Solutions |
| System Leaks | Look for the various connections, especially at the ends of the columns, and tighten the leaks. |
| Air in the Pump | Now the pressure is often unstable, high and low. More serious will cause the pump to not suck up the liquid. Open the Purge valve, flush with a flow rate of 3~5ml/min; if not, use a special syringe to suck out the air bubbles by external force at the evacuation valve. |
| No Mobile Phase Outflow | Check whether there is a mobile phase in the reservoir bottle, whether the sinker is immersed in the mobile phase and whether the pump is running. |
| Reference Valve is not Closed | Close the reference valve after reducing the flow rate. Generally, reduce to 0.1~0.2mL/min and then close the reference valve. |
Baseline drift
| Causes | Solutions |
| Column Temperature Fluctuation | Check that there are no open windows or air conditioners pointing at the column temperature chamber |
| Flow cell is Contaminated or Gassy | Flush the flow cell with methanol or other strong polar solvent (preferably with the column disconnected). If necessary, use 1N nitric acid (do not use hydrochloric acid). |
| Insufficient UV Lamp Energy | Replace with new UV lamp |
Baseline noise
| Causes | Solutions |
| Deuterium Lamp Used for too Long | Deuterium lamp should be replaced in time |
| Baseline Noise (Regular) | Air in the mobile phase, detector, or pump. | Degassing of the mobile phase is required before formal injection; flush the system to remove air from the detector or pump. |
| Leakage | Check for loose line fittings, pump leaks, salt precipitation and unusual noise, replace pump seals if necessary |
| Incomplete mixing of mobile phase | Shake by hand to mix solvents well or use low viscosity solvents to reduce variance or add a heat exchanger. |
| Column temperature too high | Reduce flow rate; adjust solvent composition of mobile phase |
| Other electronics on the same line | Disconnect other interfering electronics |
| Pump vibration | Add a pulse damper to the system if the pump is vibrating |
| Baseline Noise (Irregular) | Liquid Leakage | Check for loose fittings, pump leaks, salt precipitation and unusual noise. |
| The mobile phase is contaminated, deteriorated or made up of low quality solvents. | Check the composition of the mobile phase |
| Solvents in the mobile phase are not miscible | Select mutually soluble mobile phase |
| Contamination of flow cell | Clean the flow cell with nitric acid |
| Air bubbles in the system | Clean system with strong polar solution |
| Insufficient energy in detector lamp | Change lamp |
| Column packing lost or clogged | Replace column |
| Uneven mixing of mobile phase | Repair or replace mixer |
Peak pattern abnormality
| Causes | Solutions |
| No Peaks in the Chromatogram | The system is not fed or the sample is broken down; the pump is not infused or the mobile phase is used incorrectly; the detector is not set correctly; |
| One or Several Peaks are Negative | High absorption background of the mobile phase; air is introduced during injection; absorption of sample components is lower than that of the mobile phase |
| All Peaks are Negative | Signal cable reversed or detector output polarity reversed; optics not yet balanced. |
| All Peaks are Broad | The system is not in equilibrium; the solvent that dissolves the sample has a much lower polarity than the mobile phase; incorrect choice of column size and type; the column or guard column is contaminated or its efficiency is reduced; the effect of temperature changes. |
| Smaller Peaks than Expected | Sample viscosity is too high; sample injection fault or injection volume error; incorrect detector setting. Incorrect volume of the calibration loop; contamination of the detection cell; problems with the detector lamp. |
| Double Peaks or Shoulder Peaks | The injection volume is too large; the concentration of the sample is too high; the protection column or the column head is clogged; the protection crutch or the column is contaminated or failed; the column collapses or forms a short channel. |
Pre-extension Peaks
| High injection volume or sample concentration, the solvent that dissolves the sample is more polar than the mobile phase; contamination or failure of the guard column or chromatographic column.
Low column temperature: Increase the column temperature;
Inappropriate choice of sample solvent: use mobile phase as sample solvent;
Sample overload: reduce the sample content;
Damaged column: replace the column |
| Trailing Peaks | 1. Column overload: Reduce sample volume;
2. Increase column diameter use higher volume stationary phase;
3. Peak interference, clean and filter the sample; adjust the mobile phase;
4. Silicon hydroxylation, add triethylamine, passivate the column with alkaliphilic increases the concentration of buffer or salt decreases the mobile phase pH;
5. Dead volume or too much volume outside the column, minimise the connection point;
6. Use connecting tubing with a thin inner diameter if possible;
7. Decreased column efficiency, replace the column; |
| Flat Peaks | Incorrect detector settings; too large an injection volume or too high a sample concentration |
| Residual Peaks | 1. Adequate time to equilibrate and clean the system after each sample feed;
2. Presence of unknowns in the sample, improve sample pre-treatment;
3. Contamination of the mobile phase, replace with a new mobile phase, use it now if possible, and filter the overnight mobile phase when using it again;
4. Small air bubbles in the flow path, open the Purge valve and increase the flow rate to eliminate them. |
| Peak Bifurcation |
If the guard column or analytical column is contaminated, remove the guard column and conduct a reanalysis. If deemed necessary, replace the guard column. Sample solvent insoluble in mobile phase change sample solvent, if possible take mobile phase as sample solvent. |
| Peak Distortion | Sample overload, reduce sample volume. |
| Early Peak Distortion | 1. Inappropriate choice of sample solvent
2. Reduce sample volume
3. Use of low polarity sample solvents |
| High Tailing of Early Peaks | 1. Off-column effects
2. Adjusting system connections (using shorter, smaller ID tubing)
3. Use of small flow cell volumes |
| Additional Peaks | 1. Other components in the sample: Normal phenomenon;
2. Elution peaks from previous injection: Increase run time or gradient slope; increase flow rate;
3. Empty or ghost peaks: Check that the mobile phase is pure; use the mobile phase as the sample solvent; reduce the injection volume. |
Conclusion
Gas chromatography is suitable for approximately 20% of identified organic compounds, whereas high-performance liquid chromatography (HPLC) is required for the analysis of the remaining 80%. HPLC has the advantages of high separation efficacy, high analytical speed and good detection sensitivity. It is capable of analysing and separating thermally unstable physiologically active substances with high boiling points that cannot be gasified. At present, the separation and analysis technology of HPLC system has become the preferred technology for relevant experiments in the fields of medicine, laboratory and industry.

 English
English


